HD Medical, Inc. of Silicon Valley today announces that its flagship product, HD Steth, has received FDA clearance for all three product classification codes of DQD, DQC and DPS for Electronic Stethoscope, Phonocardiograph and Electrocardiograph combined into one device. HD Steth utilizes cutting-edge AI technology to enable clinicians to perform advanced cardiac evaluation at the point-of-care to save time and lives.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200714005235/en/
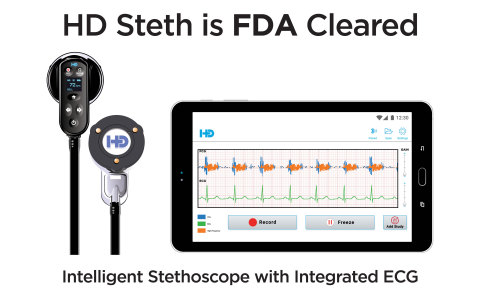
HD Steth and HD Steth App (Photo: Business Wire)
About HD Steth
HD Steth is an intelligent stethoscope that assists clinicians with capturing, recording, replaying and simultaneously visualizing heart sounds and ECG waveforms on a smart device to help detect multiple cardiac abnormalities. Patented visualization and noise cancellation technology gives HD Steth its edge. Subtle heart sounds are difficult or impossible to hear during auscultation. HD Steth delivers unsurpassed sound fidelity enhanced by visualization which allows clinicians to see the heart sounds providing instant cardiac insights.
Clinical Studies & International Sales Success
“The quality and intensity of heart sounds are phenomenal on HD Steth and it delivers the most impressive sound quality advancements in my last 40 years of stethoscope use,” said Dr. Ethiraj Raj, specializing in Cardiovascular Disease in Flint, Michigan. “The opportunity to amplify the sounds along with simultaneous visualization of heart sounds and ECG in real-time is unique. Physicians, teachers and medical students will greatly benefit from this revolutionary experience which will be a paradigm shift in cardiac auscultation. Congratulations to the HD Medical team for combining all these sensors that resulted in this exceptional instrument which will benefit the patient population as well as providers for years to come. It is truly a giant leap forward in technology.”
“HD Steth is the only stethoscope with four powerful microprocessors built into the device for advanced signal processing and intelligence, while still retaining the convenient form factor of a stethoscope,” said Shailendra Mahajan, board observer and managing director of Maxim Ventures, a major investor in HD Medical. “Components and sensors by Maxim Integrated Products, Inc. (NASDAQ: MXIM) enable low power consumption and better accuracy. The combination results in significant advantages over other products in the market.”
“Over the past year, HD Medical has been conducting multiple screening studies of over 50,000 children using HD Steth in India and has resulted in saving many lives. HD Medical has been selling internationally to medical professionals and institutions as well as domestically to veterinarians with great success. HD Medical can now market HD Steth in the U.S. and in other FDA-predicated global markets,” said Arvind Thiagarajan, founder & CEO of HD Medical. “The most intelligent stethoscope has been FDA cleared at this critically important time. During this challenging COVID-19 pandemic, HD Steth will significantly assist frontline health workers at the point-of-care detecting abnormal heart and lung sounds," he continues, welcoming the medical community to High-Fidelity Digital “HD” sound and instant visualization.
About HD Medical, Inc.
HD Medical, Inc. is a Silicon Valley-based innovator of digital health solutions for AI-enabled detection and management of cardiovascular disease (CVD). HD Steth has been awarded FDA clearance (K201299) for Product Classification Codes: DQD, DQC, DPS. The company delivers its intelligent cardiac care solutions and products globally to medical professionals and institutions as well as veterinarians through channel partners. For more information please visit www.hdmedicalgroup.com.
Note to editors: HD Steth and HD Medical are registered trademarks of HD Medical, Inc.
View source version on businesswire.com: https://www.businesswire.com/news/home/20200714005235/en/
Kristi Furrer
HD Medical, Inc.
303.525.0924
kristi@hdmedicalgroup.com
info@hdmedicalgroup.com
Permalink: http://www.businesswire.com/news/home/20200714005235/en








